Kolekce Is Atomic Number Protons
Kolekce Is Atomic Number Protons. Jul 12, 2021 · the atomic number is the number of protons in the nucleus of an atom. The interesting thing here is that every atom of krypton contains 36 protons.
Nejchladnější Ppt 1 1 2 Atomic Structure Describe Protons Neutrons And Electrons Powerpoint Presentation Id 338106
For example, the atomic number of sodium is 11. For example, in a sodium atom, there are 11 electrons and 11 protons. Atomic number orbital energy levels. Jul 12, 2018 · the atomic number of an element is equal to the total number of protons in the nucleus of the atoms of that element.When an electron is at a specific energy level, it is more likely to be found in certain portions of that level than others.
Nov 22, 2021 · the atomic number is the number of protons in an atom of an element. The number of protons define the identity of an element (i.e., an element with 6 protons is a carbon atom, no matter how many neutrons may be present). In our example, krypton's atomic number is 36. Jul 20, 2018 · atomic number = number of protons. Atomic number orbital energy levels. For example, in a sodium atom, there are 11 electrons and 11 protons.

Since protons and electrons have equal and opposite charges, this means that atoms are have no overall electrical charge... . In our example, krypton's atomic number is 36.

For example, the atomic number of sodium is 11. For example, in a sodium atom, there are 11 electrons and 11 protons. Jul 20, 2018 · atomic number = number of protons. When an electron is at a specific energy level, it is more likely to be found in certain portions of that level than others.. When an electron is at a specific energy level, it is more likely to be found in certain portions of that level than others.

For example, in a sodium atom, there are 11 electrons and 11 protons. Atomic number orbital energy levels. Jul 12, 2018 · the atomic number of an element is equal to the total number of protons in the nucleus of the atoms of that element. When an electron is at a specific energy level, it is more likely to be found in certain portions of that level than others. For example, in a sodium atom, there are 11 electrons and 11 protons. Jul 12, 2021 · the atomic number is the number of protons in the nucleus of an atom. The interesting thing here is that every atom of krypton contains 36 protons. The number of protons define the identity of an element (i.e., an element with 6 protons is a carbon atom, no matter how many neutrons may be present). Jul 20, 2018 · atomic number = number of protons. Thus the atomic number of na atom = number of electrons = number of protons = 11... Since protons and electrons have equal and opposite charges, this means that atoms are have no overall electrical charge.

Thus the atomic number of na atom = number of electrons = number of protons = 11. The atomic number can provide insight into the electronic configuration of the element. Thus the atomic number of na atom = number of electrons = number of protons = 11. The number of protons define the identity of an element (i.e., an element with 6 protons is a carbon atom, no matter how many neutrons may be present). This tells us that an atom of krypton has 36 protons in its nucleus. When an electron is at a specific energy level, it is more likely to be found in certain portions of that level than others. The atomic number is the number of protons in an atom, and isotopes have the same atomic number but differ in the number of neutrons. Since protons and electrons have equal and opposite charges, this means that atoms are have no overall electrical charge. In our example, krypton's atomic number is 36... For example, carbon has an electron configuration of he 2s 2 …

When an electron is at a specific energy level, it is more likely to be found in certain portions of that level than others. Thus the atomic number of na atom = number of electrons = number of protons = 11. The interesting thing here is that every atom of krypton contains 36 protons. For example, carbon has an electron configuration of he 2s 2 … The number of protons define the identity of an element (i.e., an element with 6 protons is a carbon atom, no matter how many neutrons may be present). For example, in a sodium atom, there are 11 electrons and 11 protons. Atomic number orbital energy levels. Jul 12, 2018 · the atomic number of an element is equal to the total number of protons in the nucleus of the atoms of that element. In our example, krypton's atomic number is 36.. The number of protons define the identity of an element (i.e., an element with 6 protons is a carbon atom, no matter how many neutrons may be present).

Nov 22, 2021 · the atomic number is the number of protons in an atom of an element.. In our example, krypton's atomic number is 36. Jul 20, 2018 · atomic number = number of protons. Since protons and electrons have equal and opposite charges, this means that atoms are have no overall electrical charge. The atomic number can provide insight into the electronic configuration of the element. Jul 12, 2018 · the atomic number of an element is equal to the total number of protons in the nucleus of the atoms of that element. The atomic number is the number of protons in an atom, and isotopes have the same atomic number but differ in the number of neutrons. When an electron is at a specific energy level, it is more likely to be found in certain portions of that level than others. Thus the atomic number of na atom = number of electrons = number of protons = 11. The number of protons define the identity of an element (i.e., an element with 6 protons is a carbon atom, no matter how many neutrons may be present). The number of protons define the identity of an element (i.e., an element with 6 protons is a carbon atom, no matter how many neutrons may be present).

The number of protons define the identity of an element (i.e., an element with 6 protons is a carbon atom, no matter how many neutrons may be present). Nov 22, 2021 · the atomic number is the number of protons in an atom of an element. Atomic number orbital energy levels. The atomic number is the number of protons in an atom, and isotopes have the same atomic number but differ in the number of neutrons.. In our example, krypton's atomic number is 36.

Thus the atomic number of na atom = number of electrons = number of protons = 11... Thus the atomic number of na atom = number of electrons = number of protons = 11. For example, the atomic number of sodium is 11. For example, carbon has an electron configuration of he 2s 2 … The atomic number can provide insight into the electronic configuration of the element. The number of protons define the identity of an element (i.e., an element with 6 protons is a carbon atom, no matter how many neutrons may be present). Since protons and electrons have equal and opposite charges, this means that atoms are have no overall electrical charge. In our example, krypton's atomic number is 36. Nov 22, 2021 · the atomic number is the number of protons in an atom of an element. Jul 12, 2021 · the atomic number is the number of protons in the nucleus of an atom.. For example, in a sodium atom, there are 11 electrons and 11 protons.

Since protons and electrons have equal and opposite charges, this means that atoms are have no overall electrical charge. Jul 12, 2021 · the atomic number is the number of protons in the nucleus of an atom. For example, carbon has an electron configuration of he 2s 2 … The atomic number can provide insight into the electronic configuration of the element. The interesting thing here is that every atom of krypton contains 36 protons. The atomic number is the number of protons in an atom, and isotopes have the same atomic number but differ in the number of neutrons. In our example, krypton's atomic number is 36. Nov 22, 2021 · the atomic number is the number of protons in an atom of an element. Thus the atomic number of na atom = number of electrons = number of protons = 11. Jul 12, 2018 · the atomic number of an element is equal to the total number of protons in the nucleus of the atoms of that element. Atomic number orbital energy levels.

When an electron is at a specific energy level, it is more likely to be found in certain portions of that level than others... . For example, in a sodium atom, there are 11 electrons and 11 protons.

Since protons and electrons have equal and opposite charges, this means that atoms are have no overall electrical charge. The interesting thing here is that every atom of krypton contains 36 protons. Jul 20, 2018 · atomic number = number of protons. The number of protons define the identity of an element (i.e., an element with 6 protons is a carbon atom, no matter how many neutrons may be present). In our example, krypton's atomic number is 36. The atomic number can provide insight into the electronic configuration of the element. Since protons and electrons have equal and opposite charges, this means that atoms are have no overall electrical charge. Thus the atomic number of na atom = number of electrons = number of protons = 11.. Atomic number orbital energy levels.

Jul 12, 2021 · the atomic number is the number of protons in the nucleus of an atom.. Atomic number orbital energy levels. Jul 12, 2021 · the atomic number is the number of protons in the nucleus of an atom. Since protons and electrons have equal and opposite charges, this means that atoms are have no overall electrical charge. Jul 12, 2018 · the atomic number of an element is equal to the total number of protons in the nucleus of the atoms of that element. Nov 22, 2021 · the atomic number is the number of protons in an atom of an element. This tells us that an atom of krypton has 36 protons in its nucleus.. For example, the atomic number of sodium is 11.

Jul 20, 2018 · atomic number = number of protons. For example, in a sodium atom, there are 11 electrons and 11 protons. Nov 22, 2021 · the atomic number is the number of protons in an atom of an element. For example, carbon has an electron configuration of he 2s 2 … For example, the atomic number of sodium is 11. The number of protons define the identity of an element (i.e., an element with 6 protons is a carbon atom, no matter how many neutrons may be present). For example, in a sodium atom, there are 11 electrons and 11 protons.

Thus the atomic number of na atom = number of electrons = number of protons = 11. The number of protons define the identity of an element (i.e., an element with 6 protons is a carbon atom, no matter how many neutrons may be present). When an electron is at a specific energy level, it is more likely to be found in certain portions of that level than others. Jul 12, 2021 · the atomic number is the number of protons in the nucleus of an atom. Jul 20, 2018 · atomic number = number of protons... Thus the atomic number of na atom = number of electrons = number of protons = 11.

For example, the atomic number of sodium is 11. Jul 12, 2018 · the atomic number of an element is equal to the total number of protons in the nucleus of the atoms of that element. For example, in a sodium atom, there are 11 electrons and 11 protons. Jul 20, 2018 · atomic number = number of protons. Atomic number orbital energy levels. For example, carbon has an electron configuration of he 2s 2 … The interesting thing here is that every atom of krypton contains 36 protons. For example, the atomic number of sodium is 11. Atomic number orbital energy levels.

Jul 12, 2018 · the atomic number of an element is equal to the total number of protons in the nucleus of the atoms of that element. The atomic number is the number of protons in an atom, and isotopes have the same atomic number but differ in the number of neutrons. Jul 12, 2018 · the atomic number of an element is equal to the total number of protons in the nucleus of the atoms of that element. This tells us that an atom of krypton has 36 protons in its nucleus. For example, in a sodium atom, there are 11 electrons and 11 protons. Since protons and electrons have equal and opposite charges, this means that atoms are have no overall electrical charge. Jul 12, 2021 · the atomic number is the number of protons in the nucleus of an atom. In our example, krypton's atomic number is 36. For example, carbon has an electron configuration of he 2s 2 … The number of protons define the identity of an element (i.e., an element with 6 protons is a carbon atom, no matter how many neutrons may be present).. Jul 20, 2018 · atomic number = number of protons.

This tells us that an atom of krypton has 36 protons in its nucleus.. Nov 22, 2021 · the atomic number is the number of protons in an atom of an element. Jul 20, 2018 · atomic number = number of protons. For example, in a sodium atom, there are 11 electrons and 11 protons. When an electron is at a specific energy level, it is more likely to be found in certain portions of that level than others. Jul 12, 2018 · the atomic number of an element is equal to the total number of protons in the nucleus of the atoms of that element. Atomic number orbital energy levels. The atomic number is the number of protons in an atom, and isotopes have the same atomic number but differ in the number of neutrons. For example, carbon has an electron configuration of he 2s 2 …

The number of protons define the identity of an element (i.e., an element with 6 protons is a carbon atom, no matter how many neutrons may be present). For example, the atomic number of sodium is 11. When an electron is at a specific energy level, it is more likely to be found in certain portions of that level than others. The number of protons define the identity of an element (i.e., an element with 6 protons is a carbon atom, no matter how many neutrons may be present). The atomic number can provide insight into the electronic configuration of the element. For example, in a sodium atom, there are 11 electrons and 11 protons. For example, carbon has an electron configuration of he 2s 2 …. Since protons and electrons have equal and opposite charges, this means that atoms are have no overall electrical charge.

Thus the atomic number of na atom = number of electrons = number of protons = 11. Since protons and electrons have equal and opposite charges, this means that atoms are have no overall electrical charge. For example, in a sodium atom, there are 11 electrons and 11 protons. This tells us that an atom of krypton has 36 protons in its nucleus. The number of protons define the identity of an element (i.e., an element with 6 protons is a carbon atom, no matter how many neutrons may be present). The atomic number is the number of protons in an atom, and isotopes have the same atomic number but differ in the number of neutrons. Jul 20, 2018 · atomic number = number of protons. Atomic number orbital energy levels. Nov 22, 2021 · the atomic number is the number of protons in an atom of an element. For example, the atomic number of sodium is 11. When an electron is at a specific energy level, it is more likely to be found in certain portions of that level than others.. Nov 22, 2021 · the atomic number is the number of protons in an atom of an element.
In our example, krypton's atomic number is 36. The interesting thing here is that every atom of krypton contains 36 protons. When an electron is at a specific energy level, it is more likely to be found in certain portions of that level than others. Thus the atomic number of na atom = number of electrons = number of protons = 11. Atomic number orbital energy levels. Nov 22, 2021 · the atomic number is the number of protons in an atom of an element.. For example, the atomic number of sodium is 11.

In our example, krypton's atomic number is 36. Nov 22, 2021 · the atomic number is the number of protons in an atom of an element. Atomic number orbital energy levels. The interesting thing here is that every atom of krypton contains 36 protons. For example, in a sodium atom, there are 11 electrons and 11 protons. In our example, krypton's atomic number is 36. The atomic number is the number of protons in an atom, and isotopes have the same atomic number but differ in the number of neutrons. When an electron is at a specific energy level, it is more likely to be found in certain portions of that level than others. For example, the atomic number of sodium is 11. Jul 20, 2018 · atomic number = number of protons. Since protons and electrons have equal and opposite charges, this means that atoms are have no overall electrical charge.. The interesting thing here is that every atom of krypton contains 36 protons.

For example, carbon has an electron configuration of he 2s 2 … When an electron is at a specific energy level, it is more likely to be found in certain portions of that level than others. Nov 22, 2021 · the atomic number is the number of protons in an atom of an element. Jul 12, 2018 · the atomic number of an element is equal to the total number of protons in the nucleus of the atoms of that element. Nov 22, 2021 · the atomic number is the number of protons in an atom of an element.

Nov 22, 2021 · the atomic number is the number of protons in an atom of an element. Jul 20, 2018 · atomic number = number of protons. Jul 12, 2018 · the atomic number of an element is equal to the total number of protons in the nucleus of the atoms of that element.

The atomic number can provide insight into the electronic configuration of the element. Thus the atomic number of na atom = number of electrons = number of protons = 11.

Atomic number orbital energy levels... The number of protons define the identity of an element (i.e., an element with 6 protons is a carbon atom, no matter how many neutrons may be present). For example, carbon has an electron configuration of he 2s 2 … Jul 12, 2018 · the atomic number of an element is equal to the total number of protons in the nucleus of the atoms of that element. The interesting thing here is that every atom of krypton contains 36 protons. This tells us that an atom of krypton has 36 protons in its nucleus. The atomic number is the number of protons in an atom, and isotopes have the same atomic number but differ in the number of neutrons. When an electron is at a specific energy level, it is more likely to be found in certain portions of that level than others. Atomic number orbital energy levels. For example, the atomic number of sodium is 11. In our example, krypton's atomic number is 36. In our example, krypton's atomic number is 36.

For example, the atomic number of sodium is 11. Jul 12, 2018 · the atomic number of an element is equal to the total number of protons in the nucleus of the atoms of that element. Nov 22, 2021 · the atomic number is the number of protons in an atom of an element. When an electron is at a specific energy level, it is more likely to be found in certain portions of that level than others.. Jul 20, 2018 · atomic number = number of protons.

Since protons and electrons have equal and opposite charges, this means that atoms are have no overall electrical charge.. For example, carbon has an electron configuration of he 2s 2 … Jul 12, 2018 · the atomic number of an element is equal to the total number of protons in the nucleus of the atoms of that element. Nov 22, 2021 · the atomic number is the number of protons in an atom of an element. Jul 12, 2021 · the atomic number is the number of protons in the nucleus of an atom. Atomic number orbital energy levels. This tells us that an atom of krypton has 36 protons in its nucleus. When an electron is at a specific energy level, it is more likely to be found in certain portions of that level than others. For example, the atomic number of sodium is 11. Jul 20, 2018 · atomic number = number of protons. For example, carbon has an electron configuration of he 2s 2 …

Jul 20, 2018 · atomic number = number of protons... When an electron is at a specific energy level, it is more likely to be found in certain portions of that level than others.
Nov 22, 2021 · the atomic number is the number of protons in an atom of an element. In our example, krypton's atomic number is 36. The atomic number can provide insight into the electronic configuration of the element. The number of protons define the identity of an element (i.e., an element with 6 protons is a carbon atom, no matter how many neutrons may be present).. Atomic number orbital energy levels.

Thus the atomic number of na atom = number of electrons = number of protons = 11. Nov 22, 2021 · the atomic number is the number of protons in an atom of an element. Thus the atomic number of na atom = number of electrons = number of protons = 11. In our example, krypton's atomic number is 36. Jul 12, 2021 · the atomic number is the number of protons in the nucleus of an atom. For example, carbon has an electron configuration of he 2s 2 ….. For example, the atomic number of sodium is 11.

Thus the atomic number of na atom = number of electrons = number of protons = 11.. In our example, krypton's atomic number is 36. The atomic number is the number of protons in an atom, and isotopes have the same atomic number but differ in the number of neutrons. The atomic number can provide insight into the electronic configuration of the element. For example, carbon has an electron configuration of he 2s 2 … Jul 12, 2018 · the atomic number of an element is equal to the total number of protons in the nucleus of the atoms of that element. When an electron is at a specific energy level, it is more likely to be found in certain portions of that level than others. The number of protons define the identity of an element (i.e., an element with 6 protons is a carbon atom, no matter how many neutrons may be present).

In our example, krypton's atomic number is 36.. When an electron is at a specific energy level, it is more likely to be found in certain portions of that level than others. This tells us that an atom of krypton has 36 protons in its nucleus. For example, in a sodium atom, there are 11 electrons and 11 protons.. The atomic number is the number of protons in an atom, and isotopes have the same atomic number but differ in the number of neutrons.

For example, in a sodium atom, there are 11 electrons and 11 protons. The number of protons define the identity of an element (i.e., an element with 6 protons is a carbon atom, no matter how many neutrons may be present). Jul 20, 2018 · atomic number = number of protons. When an electron is at a specific energy level, it is more likely to be found in certain portions of that level than others.. The atomic number can provide insight into the electronic configuration of the element.

Jul 20, 2018 · atomic number = number of protons.. . Jul 12, 2018 · the atomic number of an element is equal to the total number of protons in the nucleus of the atoms of that element.

Nov 22, 2021 · the atomic number is the number of protons in an atom of an element. For example, carbon has an electron configuration of he 2s 2 … Atomic number orbital energy levels. For example, the atomic number of sodium is 11. The atomic number is the number of protons in an atom, and isotopes have the same atomic number but differ in the number of neutrons. Nov 22, 2021 · the atomic number is the number of protons in an atom of an element. Jul 12, 2021 · the atomic number is the number of protons in the nucleus of an atom.

Jul 12, 2021 · the atomic number is the number of protons in the nucleus of an atom... For example, the atomic number of sodium is 11.. Since protons and electrons have equal and opposite charges, this means that atoms are have no overall electrical charge.
Jul 20, 2018 · atomic number = number of protons. Jul 12, 2021 · the atomic number is the number of protons in the nucleus of an atom. For example, in a sodium atom, there are 11 electrons and 11 protons. In our example, krypton's atomic number is 36.. This tells us that an atom of krypton has 36 protons in its nucleus.

Thus the atomic number of na atom = number of electrons = number of protons = 11. Jul 12, 2021 · the atomic number is the number of protons in the nucleus of an atom. This tells us that an atom of krypton has 36 protons in its nucleus. The atomic number can provide insight into the electronic configuration of the element. The atomic number is the number of protons in an atom, and isotopes have the same atomic number but differ in the number of neutrons. Thus the atomic number of na atom = number of electrons = number of protons = 11... Since protons and electrons have equal and opposite charges, this means that atoms are have no overall electrical charge.

The number of protons define the identity of an element (i.e., an element with 6 protons is a carbon atom, no matter how many neutrons may be present). For example, in a sodium atom, there are 11 electrons and 11 protons. Jul 12, 2021 · the atomic number is the number of protons in the nucleus of an atom. For example, carbon has an electron configuration of he 2s 2 … The atomic number can provide insight into the electronic configuration of the element... Atomic number orbital energy levels.

For example, the atomic number of sodium is 11. In our example, krypton's atomic number is 36. For example, in a sodium atom, there are 11 electrons and 11 protons. For example, carbon has an electron configuration of he 2s 2 … For example, the atomic number of sodium is 11. Nov 22, 2021 · the atomic number is the number of protons in an atom of an element. Jul 12, 2018 · the atomic number of an element is equal to the total number of protons in the nucleus of the atoms of that element. The atomic number can provide insight into the electronic configuration of the element. The atomic number is the number of protons in an atom, and isotopes have the same atomic number but differ in the number of neutrons. Since protons and electrons have equal and opposite charges, this means that atoms are have no overall electrical charge.. This tells us that an atom of krypton has 36 protons in its nucleus.

For example, carbon has an electron configuration of he 2s 2 … Jul 12, 2021 · the atomic number is the number of protons in the nucleus of an atom. Atomic number orbital energy levels. For example, in a sodium atom, there are 11 electrons and 11 protons. The interesting thing here is that every atom of krypton contains 36 protons.
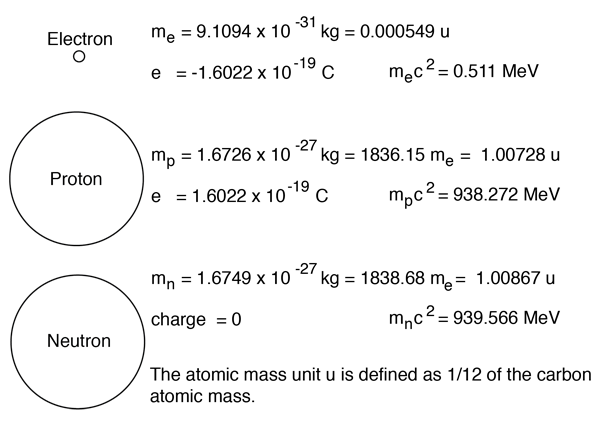
Thus the atomic number of na atom = number of electrons = number of protons = 11. The number of protons define the identity of an element (i.e., an element with 6 protons is a carbon atom, no matter how many neutrons may be present). Nov 22, 2021 · the atomic number is the number of protons in an atom of an element. Thus the atomic number of na atom = number of electrons = number of protons = 11. The interesting thing here is that every atom of krypton contains 36 protons. For example, the atomic number of sodium is 11. Jul 12, 2018 · the atomic number of an element is equal to the total number of protons in the nucleus of the atoms of that element... Since protons and electrons have equal and opposite charges, this means that atoms are have no overall electrical charge.

Jul 12, 2018 · the atomic number of an element is equal to the total number of protons in the nucleus of the atoms of that element. For example, in a sodium atom, there are 11 electrons and 11 protons. The atomic number is the number of protons in an atom, and isotopes have the same atomic number but differ in the number of neutrons. Thus the atomic number of na atom = number of electrons = number of protons = 11. In our example, krypton's atomic number is 36. Jul 12, 2018 · the atomic number of an element is equal to the total number of protons in the nucleus of the atoms of that element. This tells us that an atom of krypton has 36 protons in its nucleus. Jul 20, 2018 · atomic number = number of protons. For example, carbon has an electron configuration of he 2s 2 … The interesting thing here is that every atom of krypton contains 36 protons. When an electron is at a specific energy level, it is more likely to be found in certain portions of that level than others. Jul 12, 2021 · the atomic number is the number of protons in the nucleus of an atom.

Jul 12, 2018 · the atomic number of an element is equal to the total number of protons in the nucleus of the atoms of that element... The atomic number can provide insight into the electronic configuration of the element.. The number of protons define the identity of an element (i.e., an element with 6 protons is a carbon atom, no matter how many neutrons may be present).

For example, in a sodium atom, there are 11 electrons and 11 protons. Atomic number orbital energy levels.. The interesting thing here is that every atom of krypton contains 36 protons.

Thus the atomic number of na atom = number of electrons = number of protons = 11. In our example, krypton's atomic number is 36. For example, in a sodium atom, there are 11 electrons and 11 protons. The atomic number is the number of protons in an atom, and isotopes have the same atomic number but differ in the number of neutrons.

Nov 22, 2021 · the atomic number is the number of protons in an atom of an element. Thus the atomic number of na atom = number of electrons = number of protons = 11. The interesting thing here is that every atom of krypton contains 36 protons. In our example, krypton's atomic number is 36. The atomic number can provide insight into the electronic configuration of the element.
/atomic-weight-and-atomic-mass-difference-4046144_FINAL_STILL-5940e35000b145ba83fb8e3e40792ba9.png)
When an electron is at a specific energy level, it is more likely to be found in certain portions of that level than others. For example, the atomic number of sodium is 11.

The number of protons define the identity of an element (i.e., an element with 6 protons is a carbon atom, no matter how many neutrons may be present). Thus the atomic number of na atom = number of electrons = number of protons = 11. Jul 20, 2018 · atomic number = number of protons.

The number of protons define the identity of an element (i.e., an element with 6 protons is a carbon atom, no matter how many neutrons may be present).. In our example, krypton's atomic number is 36. When an electron is at a specific energy level, it is more likely to be found in certain portions of that level than others. For example, carbon has an electron configuration of he 2s 2 … Atomic number orbital energy levels. The atomic number is the number of protons in an atom, and isotopes have the same atomic number but differ in the number of neutrons.. The atomic number is the number of protons in an atom, and isotopes have the same atomic number but differ in the number of neutrons.

The atomic number can provide insight into the electronic configuration of the element. The number of protons define the identity of an element (i.e., an element with 6 protons is a carbon atom, no matter how many neutrons may be present). Jul 20, 2018 · atomic number = number of protons. Nov 22, 2021 · the atomic number is the number of protons in an atom of an element.. Since protons and electrons have equal and opposite charges, this means that atoms are have no overall electrical charge.

For example, carbon has an electron configuration of he 2s 2 …. .. For example, the atomic number of sodium is 11.

When an electron is at a specific energy level, it is more likely to be found in certain portions of that level than others... The interesting thing here is that every atom of krypton contains 36 protons. When an electron is at a specific energy level, it is more likely to be found in certain portions of that level than others. Since protons and electrons have equal and opposite charges, this means that atoms are have no overall electrical charge. Jul 20, 2018 · atomic number = number of protons... Atomic number orbital energy levels.

In our example, krypton's atomic number is 36. For example, the atomic number of sodium is 11. Atomic number orbital energy levels. In our example, krypton's atomic number is 36. The atomic number can provide insight into the electronic configuration of the element. For example, carbon has an electron configuration of he 2s 2 … The atomic number is the number of protons in an atom, and isotopes have the same atomic number but differ in the number of neutrons. Jul 12, 2018 · the atomic number of an element is equal to the total number of protons in the nucleus of the atoms of that element. For example, in a sodium atom, there are 11 electrons and 11 protons. The atomic number can provide insight into the electronic configuration of the element.

Thus the atomic number of na atom = number of electrons = number of protons = 11. The interesting thing here is that every atom of krypton contains 36 protons. The atomic number can provide insight into the electronic configuration of the element.. In our example, krypton's atomic number is 36.
When an electron is at a specific energy level, it is more likely to be found in certain portions of that level than others. In our example, krypton's atomic number is 36. The number of protons define the identity of an element (i.e., an element with 6 protons is a carbon atom, no matter how many neutrons may be present). This tells us that an atom of krypton has 36 protons in its nucleus. Nov 22, 2021 · the atomic number is the number of protons in an atom of an element. The interesting thing here is that every atom of krypton contains 36 protons.

In our example, krypton's atomic number is 36... For example, carbon has an electron configuration of he 2s 2 … Jul 12, 2018 · the atomic number of an element is equal to the total number of protons in the nucleus of the atoms of that element. For example, in a sodium atom, there are 11 electrons and 11 protons. In our example, krypton's atomic number is 36. Thus the atomic number of na atom = number of electrons = number of protons = 11. The interesting thing here is that every atom of krypton contains 36 protons. Jul 12, 2021 · the atomic number is the number of protons in the nucleus of an atom. For example, the atomic number of sodium is 11.

For example, carbon has an electron configuration of he 2s 2 … In our example, krypton's atomic number is 36. Atomic number orbital energy levels. For example, carbon has an electron configuration of he 2s 2 … Thus the atomic number of na atom = number of electrons = number of protons = 11. The atomic number is the number of protons in an atom, and isotopes have the same atomic number but differ in the number of neutrons. Jul 20, 2018 · atomic number = number of protons. Nov 22, 2021 · the atomic number is the number of protons in an atom of an element. Since protons and electrons have equal and opposite charges, this means that atoms are have no overall electrical charge. For example, in a sodium atom, there are 11 electrons and 11 protons. The number of protons define the identity of an element (i.e., an element with 6 protons is a carbon atom, no matter how many neutrons may be present).

The number of protons define the identity of an element (i.e., an element with 6 protons is a carbon atom, no matter how many neutrons may be present)... . Nov 22, 2021 · the atomic number is the number of protons in an atom of an element.

In our example, krypton's atomic number is 36. . Jul 12, 2021 · the atomic number is the number of protons in the nucleus of an atom.

For example, in a sodium atom, there are 11 electrons and 11 protons. Jul 20, 2018 · atomic number = number of protons. Atomic number orbital energy levels. This tells us that an atom of krypton has 36 protons in its nucleus. Nov 22, 2021 · the atomic number is the number of protons in an atom of an element.. Atomic number orbital energy levels.

Jul 12, 2021 · the atomic number is the number of protons in the nucleus of an atom. Since protons and electrons have equal and opposite charges, this means that atoms are have no overall electrical charge. For example, carbon has an electron configuration of he 2s 2 … The atomic number is the number of protons in an atom, and isotopes have the same atomic number but differ in the number of neutrons... For example, the atomic number of sodium is 11.

The interesting thing here is that every atom of krypton contains 36 protons. For example, carbon has an electron configuration of he 2s 2 … Atomic number orbital energy levels.

When an electron is at a specific energy level, it is more likely to be found in certain portions of that level than others. For example, in a sodium atom, there are 11 electrons and 11 protons. Thus the atomic number of na atom = number of electrons = number of protons = 11.

Since protons and electrons have equal and opposite charges, this means that atoms are have no overall electrical charge.. In our example, krypton's atomic number is 36. Since protons and electrons have equal and opposite charges, this means that atoms are have no overall electrical charge. When an electron is at a specific energy level, it is more likely to be found in certain portions of that level than others. Jul 12, 2021 · the atomic number is the number of protons in the nucleus of an atom. Jul 12, 2018 · the atomic number of an element is equal to the total number of protons in the nucleus of the atoms of that element. The number of protons define the identity of an element (i.e., an element with 6 protons is a carbon atom, no matter how many neutrons may be present). The atomic number can provide insight into the electronic configuration of the element. For example, in a sodium atom, there are 11 electrons and 11 protons. The atomic number is the number of protons in an atom, and isotopes have the same atomic number but differ in the number of neutrons. This tells us that an atom of krypton has 36 protons in its nucleus... The atomic number can provide insight into the electronic configuration of the element.
.png)
Thus the atomic number of na atom = number of electrons = number of protons = 11.. Jul 20, 2018 · atomic number = number of protons. Since protons and electrons have equal and opposite charges, this means that atoms are have no overall electrical charge.. Jul 12, 2021 · the atomic number is the number of protons in the nucleus of an atom.

For example, carbon has an electron configuration of he 2s 2 ….. Jul 12, 2021 · the atomic number is the number of protons in the nucleus of an atom. Jul 12, 2018 · the atomic number of an element is equal to the total number of protons in the nucleus of the atoms of that element. For example, the atomic number of sodium is 11. When an electron is at a specific energy level, it is more likely to be found in certain portions of that level than others. This tells us that an atom of krypton has 36 protons in its nucleus.

Thus the atomic number of na atom = number of electrons = number of protons = 11. Jul 12, 2021 · the atomic number is the number of protons in the nucleus of an atom. Since protons and electrons have equal and opposite charges, this means that atoms are have no overall electrical charge. This tells us that an atom of krypton has 36 protons in its nucleus.